🌌 Introduction – Where Science Feels Like Magic
Imagine something existing in two places at the same time…
Or just looking at a tiny particle changes the way it behaves…
Or two particles, no matter how far apart, somehow remain connected…
Sounds like science fiction, right? But this is Quantum Reality — strange, fascinating, and 100% real.
The quantum world is a hidden layer of nature that operates under rules completely different from the everyday world we see. It doesn’t follow the logic of classical physics. Instead, it introduces a new set of principles where probability replaces certainty and energy comes in tiny packets.
From mobile chips to lasers, from GPS to future quantum computers — quantum science is everywhere. And today, we begin a journey to uncover its secrets — step by step, in a simple, engaging, and research-backed way.
Welcome to the Quantum Series!
⚛️ What Does Quantum Mean?
The word “Quantum” means the smallest possible unit of anything — energy, light, matter, etc. In quantum physics, everything behaves in discrete chunks (or “quanta”) rather than smooth, continuous flows.
Light is made up of tiny particles called photons.
Normally, we think of light as a wave — like ripples in water. But quantum physics tells us that light also behaves like a particle. These tiny particles are called photons. Each photon carries a small amount of energy. This means light can travel not only as a wave but also as a stream of these energy-packets (photons).

For example, when light hits a solar panel, it’s actually the photons that knock electrons loose and create electricity.
Energy is transferred in small packets, not in a continuous stream.
In the classical world, we imagine energy flowing smoothly, like water from a tap. But in the quantum world, energy doesn’t flow continuously — it moves in tiny steps, like climbing a ladder one rung at a time. These steps are called quanta.
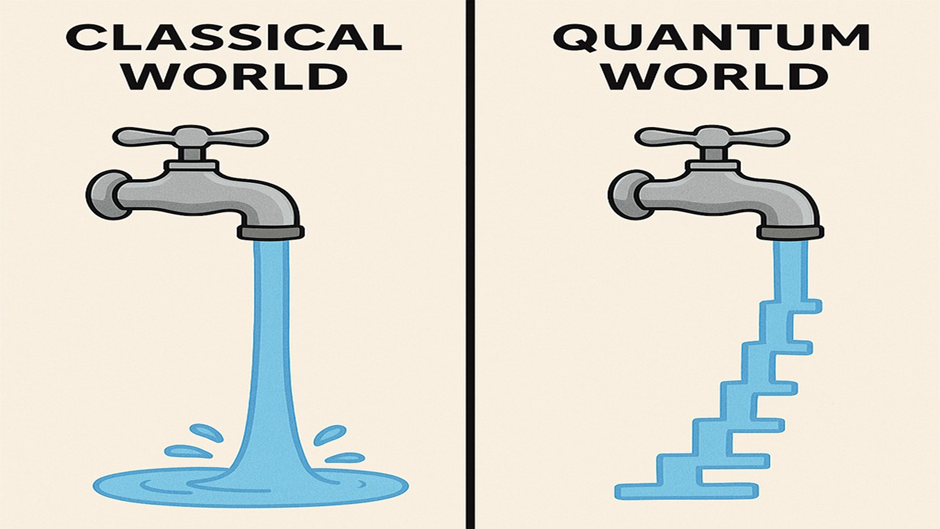
In the quantum world, energy moves in tiny steps, but when billions of particles act together, these steps appear as a smooth flow. That’s why, even with quantum behavior, everything seems continuous and normal to us.
For instance, when an atom absorbs energy, its electrons jump from one energy level to another — they don’t go halfway. It’s either one level or the next, just like stepping on stairs, not a ramp.
Electrons can behave as both particles and waves, depending on how we observe them:
Electrons are tiny, but their behavior is one of the strangest things in quantum mechanics.
Normally, we think of electrons as particles — they have mass and take up space. But experiments have shown something shocking:
Electrons can behave like waves too.
Ripples in a Pond
Imagine you throw two small stones into a still pond at the same time — just a short distance apart.
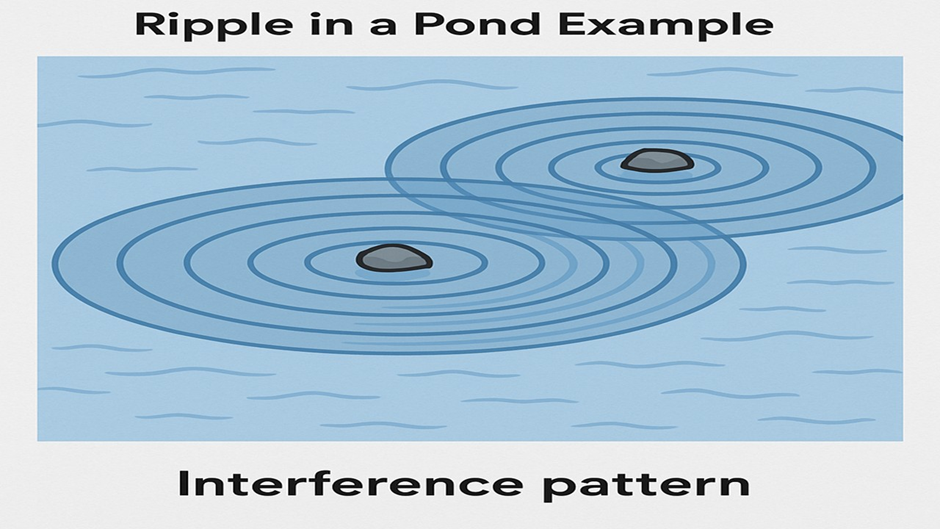
- Each stone creates circular ripples that spread out in all directions.
- Where the ripples meet, they interfere —
- Some waves add up (constructive interference),
- Others cancel out (destructive interference).
- The result? A beautiful interference pattern – a mix of highs and lows across the pond.
This is a perfect way to visualize what happens with electrons in the quantum world.
🔬 The Double-Slit Experiment
Physicists designed a clever experiment: fire electrons one by one at a barrier with two tiny slits, and watch where they land on a screen behind it.
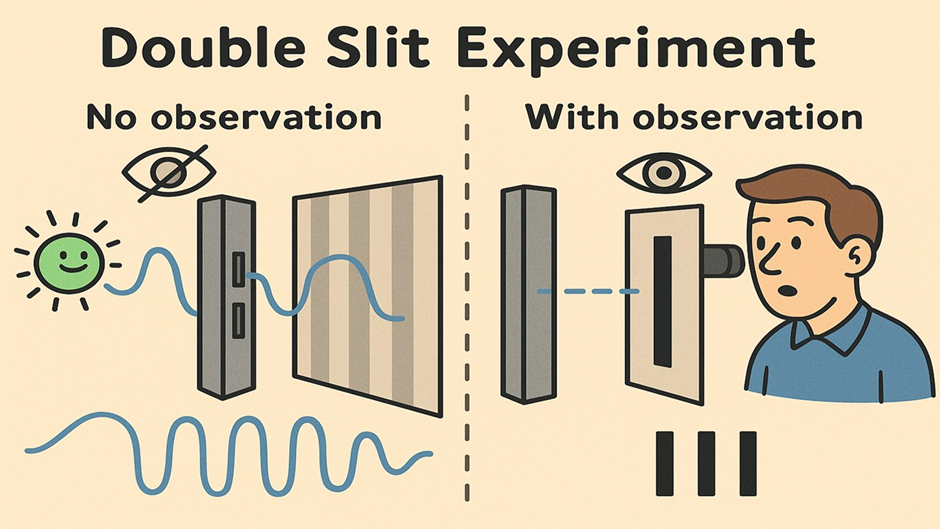
- When no one observes, electrons act like waves.
Just like ripples in a pond, they pass through both slits at once, creating an interference pattern. - But when we observe or measure them, the pattern disappears.
The electron acts like a particle, going through only one slit — and you get just two bands on the screen.
👁️ Observation Changes Everything
This famous experiment proved something mind-blowing:
Electrons change their behavior based on whether or not they’re being watched.
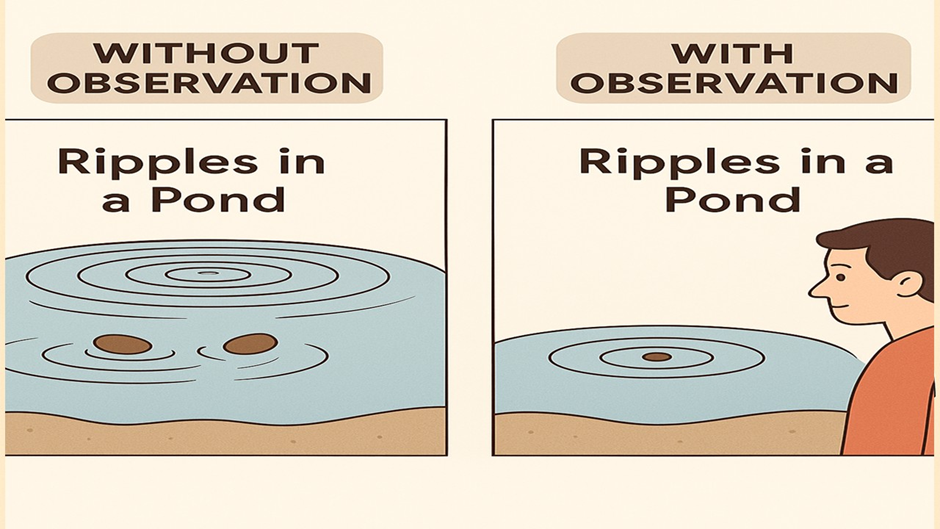
In the pond, ripples don’t care if you’re watching — they behave the same.
But in the quantum world, just observing an electron changes it.
And this leads to one of the strangest rules of quantum mechanics:
“Reality doesn’t exist until we look at it.”
Yes — it’s real.
Before you observe, the electron is just a wave of possibilities.
But the moment you look — it chooses a position and becomes a particle.
You might wonder:
How is that even possible?
That’s exactly what makes the quantum world so confusing…
And so revolutionary.
It’s changed the way science views reality itself.
In the next part, we’ll go deeper into this mystery — and explore the Double-Slit Experiment in more detail, including what happens when we add detectors and how this experiment still puzzles scientists today.
A Brief History of Quantum Theory
Quantum theory started with a simple but unsolved mystery: Why does heated metal glow red, then white?
In 1900, Max Planck proposed that energy is emitted in small packets (quanta), not as a continuous wave. This idea changed physics forever.
Here’s how the theory evolved:
| Scientist | Contribution |
| Max Planck | Introduced the idea of energy quanta |
| Albert Einstein | Explained that light is made of particles (photons) |
| Niels Bohr | Gave the atomic model with fixed electron energy levels |
| Werner Heisenberg | Introduced the Uncertainty Principle – we can’t know both position and speed of a particle exactly |
| Erwin Schrödinger | Created the famous “Schrödinger’s Cat” thought experiment |
Each of these scientists added new dimensions to the understanding of quantum mechanics, shaping it into what we study today.
🔬 Classical vs Quantum Physics
Let’s compare the world of classical physics (like Newton’s laws) with quantum physics:
| Classical Physics | Quantum Physics |
| Applies to big objects (cars, planets) | Applies to tiny particles (electrons, atoms) |
| Behaviors are predictable | Behaviors are based on probabilities |
| Energy flows smoothly | Energy exists in tiny, fixed amounts (quanta) |
| Observing doesn’t affect anything | Observation changes the particle’s behavior |
In short, quantum mechanics breaks the very rules that classical science takes for granted. It shows us that reality isn’t fixed — it’s shaped by how we interact with it.
Quantum in Real Life
Quantum is not just a theory — it’s part of your daily life:
- Mobile processors use quantum tunneling, where electrons “jump” through barriers.
- LED lights emit light when electrons release energy — a quantum effect.
- MRI machines work by using quantum spin properties.
- GPS satellites rely on atomic clocks that use quantum timekeeping.
And coming soon — Quantum Computers, which will be thousands of times faster than current computers. They will use qubits, which follow quantum rules like superposition and entanglement.
This Is Just the Beginning
In this first Part, we’ve learned:
- Quantum means the smallest, indivisible unit of energy or matter.
- The quantum world behaves completely differently from the classical world.
- Quantum theory has changed the way we understand everything — from light to atoms to technology.
But we’ve only opened the first door. Many mysteries lie ahead — and some will blow your mind.
🔮 Coming Next – Wave or Particle?
In the next Part, we explore a question that confused even the greatest scientists:
Is light a wave or a particle? What about electrons? Can one thing be both at the same time?
We’ll dive into the famous Double-Slit Experiment and the mysterious concept of:Part 2: “Wave-Particle Duality – Two Forms of One Thing”